
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.14, Problem 13.32P
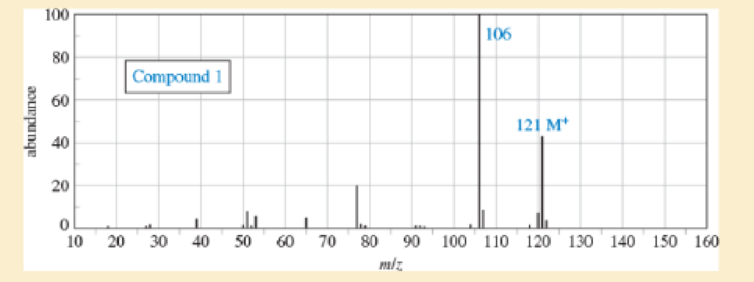
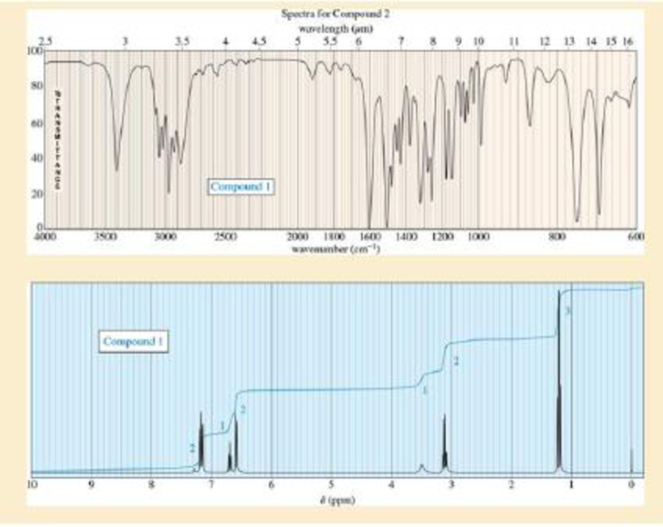
Sets of spectra are given for two compounds. For each set,
- A. look at each spectrum individually, and list the structural characteristics you can determine from that spectrum.
- B. look at the set of spectra as a group, and propose a tentative structure.
- C. verify that your proposed structure accounts for the major features of each spectrum. The soluton for compound 1 is given after the problem, but go as far as you can before looking at the solution.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
From the spectra A-J and in the NMR Spectra tile, select the letter that corresponds to 1. methyl butanoate2. benzaldehyde3. 1-chlorobutane4. 1-chloro-2-methylpropane5. butan-2-one6. propan-2-ol7. propanal
3. Look at the two spectra below, one of which is 2-methylcyclohexanol and one of which is
3methylcyclohexene. Which spectrum belongs to which compound? Explain your reasoning.
10-
4000
200
3500
3000
2500
1500
1000
600
Spectrum A
90
20
30
20
10
500
3000
2500
2000
1600
1000
500
Vieters kn1
Spectrum B
ORGANIC CHEMISTRY
PLEASE EXPLAIN, SEE PHOTO: Predict the structure for this unknown. Please provide relevant information regarding each peak and the rationale for how you came to identify each structure. Label any and all peaks. Show all work.
Chapter 13 Solutions
Organic Chemistry (9th Edition)
Ch. 13.5A - In a 300-MHz spectrometer, the protons in...Ch. 13.5B - Prob. 13.2PCh. 13.6 - Determine the number of different kinds of protons...Ch. 13.6 - Prob. 13.4PCh. 13.7 - Draw the integral trace expected for the NMR...Ch. 13.7 - Prob. 13.6PCh. 13.8C - Draw the NMR spectra you would expect for the...Ch. 13.8D - Draw the NMR spectra you expect for the following...Ch. 13.8D - a. Assign protons to the peaks in the NMR spectrum...Ch. 13.8D - Prob. 13.10P
Ch. 13.8D - Two spectra are shown. Propose a structure that...Ch. 13.9 - Prob. 13.12PCh. 13.9 - The spectrum of trans-hex-2-enoic acid follows. a....Ch. 13.9 - Prob. 13.14PCh. 13.9 - Prob. 13.15PCh. 13.10 - Prob. 13.16PCh. 13.10 - If the imaginary replacement of either of two...Ch. 13.10 - Predict the theoretical number of different NMR...Ch. 13.11B - Prob. 13.19PCh. 13.11B - Prob. 13.20PCh. 13.11B - Prob. 13.21PCh. 13.11B - Prob. 13.22PCh. 13.11B - Prob. 13.23PCh. 13.11B - Prob. 13.24PCh. 13.12E - Draw the expected broadband-decoupled 13 C N M R...Ch. 13.12E - a. Show which carbon atoms correspond with which...Ch. 13.12E - Repeat Problem13-25, sketching the...Ch. 13.12F - Prob. 13.28PCh. 13.13 - A bottle of allyl bromide was found to contain a...Ch. 13.13 - A laboratory student was converting cyclohexanol...Ch. 13.14 - Sets of spectra are given for two compounds. For...Ch. 13 - An unknown compound has the molecular formula C 9...Ch. 13 - Prob. 13.34SPCh. 13 - Predict the approximate chemical shifts of the...Ch. 13 - Prob. 13.36SPCh. 13 - Prob. 13.37SPCh. 13 - Prob. 13.38SPCh. 13 - Prob. 13.39SPCh. 13 - Prob. 13.40SPCh. 13 - For each compound shown below. 1. sketch the 13 C...Ch. 13 - Prob. 13.42SPCh. 13 - Prob. 13.43SPCh. 13 - Prob. 13.44SPCh. 13 - Prob. 13.45SPCh. 13 - Prob. 13.46SPCh. 13 - A compound was isolated as a minor constituent in...Ch. 13 - Prob. 13.48SPCh. 13 - The three isomers of dimethylbenzene are commonly...Ch. 13 - a. Draw all six isomers of formula C 4 H 8...Ch. 13 - Prob. 13.51SPCh. 13 - Hexamethylbenzene undergoes free-radical...Ch. 13 - Each of these four structures has molecular...Ch. 13 - Prob. 13.54SPCh. 13 - Phenyl Grignard reagent adds to 2-methylpropanal...Ch. 13 - Prob. 13.56SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. Analyze the two IR’s below. One corresponds to 2,3-dimethyl-1-butene, and the other correspondsto 2,3-dimethyl-2-butene.a. Draw the structures of both molecules.b. Match each compound to the correct spectrum.c. Explain how you arrived at your selection. In your explanation you should state at least tworeasons (e.g. peaks that are missing/present, strong vs weak, symmetry vs. asymmetry) andyou should support your reasons with data from the IR spectra.arrow_forwardDetermine the structure of this molecule using the NMR. Please draw the structure and explain how you got it. Thank you for your help!arrow_forward1. NMR analysis: Draw the full structure of diphenylmethanol. Show all bonds to the hydrogens. Label the different sets of hydrogens (i.e. Hạ. Hp, H1, H2, etc) based on which ones are equivalent and which ones are not. Assign each set of hydrogens with a specific signal on the spectrum. Create a table that summarizes: the chemical shift, integration, multiplicity, proton assignment, and justification of each signal.arrow_forward
- 1.) Use the H - NMR spectrum to determine how many different types of hydrogens are present in the unknown molecule b.) Analyze the 1H - NMR chemical shifts to determine the functional groups that may be present in your molecule. Also, label the splitting pattern type (singlet, doublet, triplet, quartet, multiplet etc) for each signal of your 1H - NMR spectrumarrow_forward1) Draw the structure of your unknown below and assign the spectrum. That is, label each different peak in the spectrum with a letter (e.g. a, b, c, etc.) and place the letter on the structure you drew near the hydrogens or sets of hydrogens responsible for the peak. See the experiment instructions for a discussion of how to assign a spectrum and a worked example.arrow_forward5. Propose structures for the following molecules using their NMR spectra and empirical formulas. Explain the choice. F. Assign the lines in NMR spectrum to their respective protons in 4-methoxybenzaldehyde. Hint: Draw a structure showing all atoms, this will help to identify the types of protonsarrow_forward
- How many different 'H signals appear for this compound? OA. A. 10 OB. 9 O C. 8 OD. 7 OE.6arrow_forwardHow many different carbon peaks are in the proton-NMR spectrum of tamoxifen (image below)? Note, look at the molecular structure and NMR spectra to determine this number. Discuss why you came to this conclusion and draw pictures/ label to help explain.arrow_forwardTrying to figure out the structure based on this NMR. unsat=4 so probably includes benzene ring. Unsure of how substitued the ring is.arrow_forward
- 4. Propose a structure (Lewis structure okay) with molecular formula C13H18O2 that is consistent with the ¹H-NMR spectrum below. Place your structure in the box and label each hydrogen atom using the corresponding labels given in the spectrum (a, b, c, etc.). Note that the peak labeled "TMS" at 0 ppm is not part of your proposed structure and should not be included. Hints: the MS of this molecule has major fragment peaks at m/z = 57 and 73; calculate the IHD for the given molecular formula. arbitrary intensity 4. Draw structure and label hydrogen atoms a br s 1H 12 115 11 105 10 95 85 b c d 2H d 2H *** 'ક 6 pom 35 P q 1H e d 2H g d 3H f m 1H h d 6H 05 TMS 35arrow_forwardFor each of the ten spectra you should determine which peaks are the most important for identification purposes, what their wavenumber values are, and what specific bonds and/or functional groups they indicate. Record those in the spaces under each spectrum. You don’t have to use each space. Then, from the list of possible compounds, identify what compound is responsible for each spectrum. Realize that these are all of real samples and there may be impurities present that cause weak peaks which would not normally be caused by the compound, such as the one at 3450 cm-1 in Spectrum 1.arrow_forwardChemistry 1.Predict the IR signal in the 1-bromo-3-ethylbenzene. (Approximate wave number for each Functional groups) 2.Predict the 1HNMR signal in the 1-bromo-3-ethylbenzene. (No of signals, splitting pattern of signals, integration, etc)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY