
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 28, Problem 28.7P
Interpretation Introduction
Interpretation:
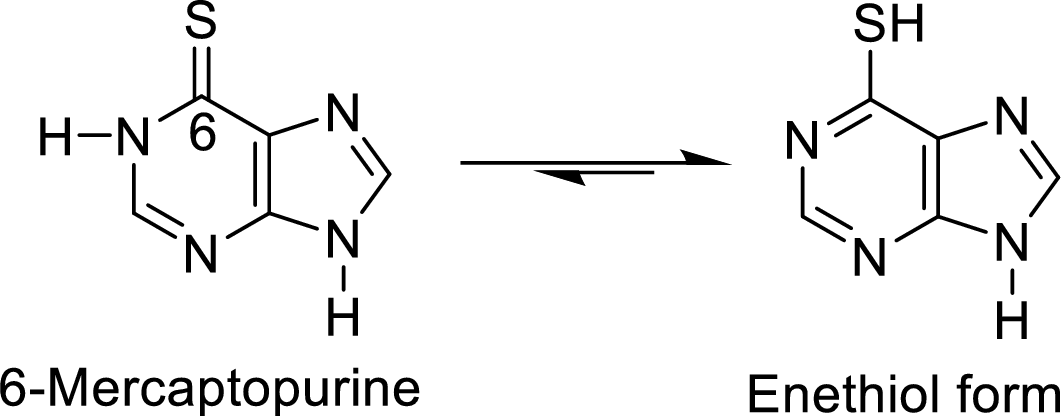
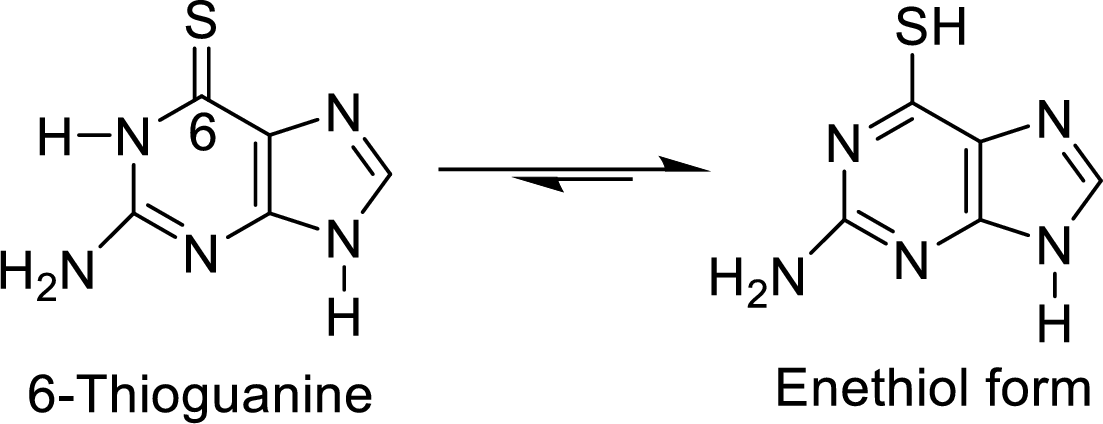
The structural formulas for the enethiol forms of 6-mercaptopurine and 6-thioguanine has to be drawn.
Concept Introduction:
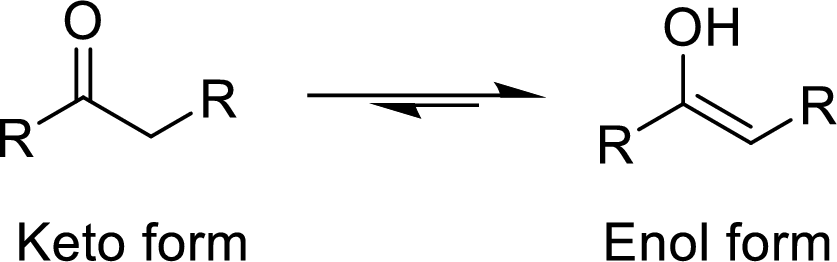
The
Expert Solution & Answer
Explanation of Solution
The enethiol is also the enol form which contains Sulphur molecule instead of Oxygen. The enethiol form is the alcoholic form of the Sulphur. The double bonded Sulphur is converted to thiol form of the Sulphur.
The enethiol form of 6-mercaptopurine is
The enethiol form of 6-thioguanine is
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
A pioneer in designing and synthesizing antimetabolites that could destroy cancer cells
was George Hitchings at the Burroughs Wellcome Company. In 1942, he initiated a pro-
gram to discover DNA antimetabolites, and in 1948, he and Gertrude Elion synthesized
6-mercaptopurine, a successful drug for treating acute leukemia. Another DNA antime-
tabolite synthesized by Hutchings and Elion was 6-thioguanine. Hitchings and Elion along
with Sir James W. Black won the 1988 Nobel Prize in Physiology or Medicine for their
discoveries of "important principles of drug treatment." In each drug, the oxygen at carbon
6 of the parent molecule is replaced by divalent sulfur. Draw structural formulas for the
enethiol (the sulfur equivalent of an enol) forms of 6-mercaptopurine and 6-thioguanine.
HN
HN
H,N
H
6-Mercaptopurine
6-Thioguanine
264&OpenVellumHMAC-ab644d9786730f4650f72638a7bd0c5b#10001
Part A
L
Submit
Part B
amylose and cellulose
Match the words in the left column to the appropriate blanks in the sentences on the right.
a(16)
B(14)
a(12)
glucose
a(14)
8(16)
a(12)
fructose
Request Answer
a(12)
B(16)
a(16)
cellulose and chitin
Match the words in the left column to the appropriate blanks in the sentences on the right.
glucose
-tose also called - Search
a(14)
fructose
8(14)
B(12)
x | +
Both are linear polysaccharides composed entirely of
linkages are
but in cellulose, they are
Both are linear polysaccharides with
2
DELL
and chitin contains N-acetylglucosamine.
A Q
Reset Help
In amylose, the glycosidic
glycosidic bonds. Cellulose contains
he
Reset Help
ENG
Constants
4) Paxlovid is treatment for COVID-19 that was recently approved for use because it can
drastically reduce the risk for hospitalization and death when taken early in the
infection. It is actually a package of two different antiviral drugs. One of those is
Nirmatrelvir, which is a modified tripeptide protein mimetic designed to covalently bind
to and inhibit the main protease (MPro) from the SARS-CoV-2 virus. This protease
recognizes and hydrolyzes peptide bonds at specific points in their sequence, and it is
essential for viral replication.
a. Below is one of the sequences that Mpro recognizes and cleaves. It cleaves the peptide bond between
the glutamine and the glycine. Draw out the structure of a peptide with this sequence, including the
appropriate stereochemistry for biological amino acids and assuming the peptide is at physiological
pH (pH = 7.4).
SGVTFQGKF
b. What would the net charge of the peptide be at physiological pH?.
c. At what pH would the peptide be net neutral?
Chapter 28 Solutions
Organic Chemistry
Ch. 28.1 - Prob. 28.1PCh. 28.2 - Prob. 28.2PCh. 28.2 - Prob. 28.3PCh. 28.3 - Here is a portion of the nucleotide sequence in...Ch. 28.4 - The following section of DNA codes for oxytocin, a...Ch. 28.5 - Prob. 28.6PCh. 28 - Prob. 28.7PCh. 28 - Following are structural formulas for cytosine and...Ch. 28 - Prob. 28.9PCh. 28 - Prob. 28.10P
Ch. 28 - Prob. 28.11PCh. 28 - Prob. 28.12PCh. 28 - Prob. 28.13PCh. 28 - Prob. 28.14PCh. 28 - Prob. 28.15PCh. 28 - Draw a structural formula of the DNA...Ch. 28 - List the postulates of the Watson-Crick model of...Ch. 28 - Prob. 28.18PCh. 28 - Prob. 28.19PCh. 28 - Prob. 28.20PCh. 28 - Prob. 28.21PCh. 28 - Prob. 28.22PCh. 28 - Prob. 28.23PCh. 28 - Prob. 28.24PCh. 28 - Write the DNA complement for 5-ACCGTTAAT-3. Be...Ch. 28 - Prob. 28.26PCh. 28 - Prob. 28.27PCh. 28 - Compare DNA and RNA is these ways. (a)...Ch. 28 - What type of RNA has the shortest lifetime in...Ch. 28 - Prob. 28.30PCh. 28 - Prob. 28.31PCh. 28 - Prob. 28.32PCh. 28 - Write the mRNA codons for the following. (a)...Ch. 28 - Prob. 28.34PCh. 28 - Prob. 28.35PCh. 28 - Prob. 28.36PCh. 28 - Prob. 28.37PCh. 28 - Prob. 28.38PCh. 28 - Prob. 28.39PCh. 28 - What polypeptide is coded for by this mRNA...Ch. 28 - The alpha chain of human hemoglobin has 141 amino...Ch. 28 - Prob. 28.42P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q23: Dr. Alghe has just performed a restriction digest on DNA she extracted from her samples. She must now run her DNA out on a gel to separate the fragments based on size. To do this she must prepare a 1.1% agarose gel, where the percentage is determined as mass/vol. How many grams of agarose must she add to 413 mL of buffer in order to arrive at the correct percentage? (answer to 2 decimals) **Assume the dissolution of agarose will not alter the final volume of the solution**arrow_forwardWhat is the sequence of bases in the strand of DNA that is complementary to the one shown below? 5'-T-T-C-A-C-T-G-A-C-3' a.) 3'-U-U-C-A-C-U-G-A-C-5' b.) 3'-T-T-C-A-C-T-G-A-C-5' c.) 5'-A-A-G-T-G-A-C-T-G-3' d.) 5'-T-T-C-A-C-T-G-A-C-3' e.) 5'-G-T-C-A-G-T-G-A-A-3'arrow_forwardIdentify the following antioxidant molecule. A. alpha tocopherol hydroquinone B.dehydroascorbic acid CH3 CH3 C. alpha tocopherol semi-quinone \ / D. ascorbic acid C = C E. alpha tocopherol quinone / \ : O – C C – O CH3 \\ // \ / C – C C – CH2–CH2–CH2–CH–CH2–CH2–CH2–CH–CH2–CH2–CH2–CH–CH3 / \ / l l l CH3 CH2– CH2…arrow_forward
- 2.70 Why do you think an inhibitor molecule is needed to induce the polymerization of ethylene?arrow_forwardThere are 41 = 4 mononucleotides of DNA, there are 42 = 16 possible dinucleotides, and so on. If a segment of DNA were completely random, how many nucleotides long would it need to be in order to have one possible sequence for each person on Earth (currently about 7.5 billion people)?arrow_forwardDNA Replication1) Construct the complementary strand of DNA (strand II) that would pair with the DNA strand I sequence below. Remember that nucleotides are always added in the 5 prime to 3 prime direction. The base pairing rules are: DNA DNAadenine (A) always bonds with thymine (T) thymine (T) always bonds with adenine (A) guanine (G) always bonds with cytosine (C) cytosine (C) always bonds with guanine (G) STRAND I: 3’ T A C T A T A G T C T G T C T C C C A C T 5’ STRAND II: 5’ 3’arrow_forward
- The DNA of bacteriophage lambda has 1.2 x 10^5 nucleotides. How many proteins of molecular weight 40,000 could be coded by this DNA? Assume a molecular weight of 100 for the average amino acid.arrow_forwardWhy are Guanidins and Amidins such strong bases?arrow_forwardSection one. Match enzyme or molecule with its function in bacteria. Answers may be used more than once or not at all. DNA ligase d. tRNA promoter а. g. b. DNA helicase 1RNA h. terminator е. RNA polymerase f. DNA polymerase III i. stop codon с. 37. main enzyme in catalyzing DNA → mRNA transcription 38. unwinds double-stranded DNA at the replication fork 39. Where transcription stops 40. adds new DNA bases in the 5'→3’ direction 41. the "translator" molecule 42. contains codons 43. contains anti-codonsarrow_forward
- Compare the two peptides shown below, which can be ordered from chemical supply companies: Time left 0:58:26 H₂N NH OH O b. O c. NH. CH3 Which statement is correct about these peptides? OH Peptide A Peptide B O a. Peptide A can only be in an alpha helix and cannot form a beta-strand; Peptide B can be in alpha helix or beta strand Peptide B will have a higher absorbance at 280nm because it is shorter in length Peptide A will have a higher absorbance at 280nm because it has more aromatic amino acids O d. Both peptide A and peptide B lack any acidic amino acidsarrow_forwardplease send me mcqsarrow_forwardThe image below is a positive ionization mode Electrospray ionization-mass spectrum (ESI-MS) spectrum for a peptide, obtained using high resolution mass spectrometry. If ionization occurs through de-protonation (assuming that the mass of a proton is 1.0 Da), what is the neutral mass of the peptide, in Da? 627.271 627.604 100 - 627.938 628.271 628.604 628.938 627.0 628.0 629.0 mass/charge (m/z) 628.271 626.271 1878.813 O 1884.813 % Abundancearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY