
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 90AP
Two possible Lewis diagrams for sulfine
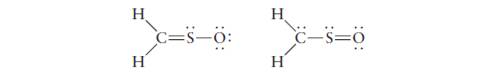
(a) Compute the formal charges on all atoms.
(b) Draw a Lewis diagram for which all the atoms in sulfine have formal charges of zero.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN.
(a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom.
(b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule— namely, NSF, which has a central sulfur atom?
(c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain. 100. The gas
Two possible Lewis diagrams for sulfine (H2CSO) are
H
H.
c=s-ö:
C-s=0
H
H
(a) Compute the formal charges on all atoms.
(b) Draw a Lewis diagram for which all the atoms in
sulfine have formal charges of zero.
The hypochlorite ion, ClO-, is the active ingredient inbleach. The perchlorate ion, ClO4-, is a main componentof rocket propellants. Draw Lewis structures for both ions.
(a) What is the formal charge of Cl in the hypochlorite ion?(b) What is the formal charge of Cl in the perchlorate ion, assumingthe Cl—O bonds are all single bonds? (c) What is theoxidation number of Cl in the hypochlorite ion? (d) Whatis the oxidation number of Cl in the perchlorate ion, assumingthe Cl—O bonds are all single bonds? (e) In a redox reaction,which ion would you expect to be more easily reduced?
Chapter 3 Solutions
Principles of Modern Chemistry
Ch. 3 - Before the element scandium was discovered in...Ch. 3 - Prob. 2PCh. 3 - Prob. 3PCh. 3 - Prob. 4PCh. 3 - Prob. 5PCh. 3 - A gold nucleus is located at the origin of...Ch. 3 - Prob. 7PCh. 3 - A gold nucleus is located at the origin of...Ch. 3 - Prob. 9PCh. 3 - Prob. 10P
Ch. 3 - Use the data in Table 3.1 to plot the logarithm of...Ch. 3 - Use the data in Table 3.1 to plot the logarithm of...Ch. 3 - Prob. 13PCh. 3 - Prob. 14PCh. 3 - Prob. 15PCh. 3 - Prob. 16PCh. 3 - Prob. 17PCh. 3 - Prob. 18PCh. 3 - HF has equilibrium bond length of 0.926 A and bond...Ch. 3 - Prob. 20PCh. 3 - For each of the following atoms or ions, state the...Ch. 3 - Prob. 22PCh. 3 - Use the data in Figure 3.11 and Table 3.2 to...Ch. 3 - Use the data in Figure 3.11 and Table 3.2 to...Ch. 3 - Prob. 25PCh. 3 - In a gaseous RbF molecule, the bond length is...Ch. 3 - The bond lengths of the XH bonds in NH3,PH3 , and...Ch. 3 - Arrange the following covalent diatomic molecules...Ch. 3 - The bond length in HI(1.62) is close to the sum of...Ch. 3 - Prob. 30PCh. 3 - Use electronegativity values to arrange the...Ch. 3 - Use electronegativity values to rank the bonds in...Ch. 3 - Prob. 33PCh. 3 - Prob. 34PCh. 3 - Prob. 35PCh. 3 - Estimate the percent ionic character of the bond...Ch. 3 - The percent ionic character of a bond can be...Ch. 3 - The percent ionic character of the bonds in...Ch. 3 - Assign formal charges to all atoms in the...Ch. 3 - Assign formal charges to all atoms in the...Ch. 3 - Determine the formal charges on all the atoms in...Ch. 3 - the formal charges on all the atoms in the...Ch. 3 - Prob. 43PCh. 3 - In each of the following Lewis diagrams, Z...Ch. 3 - Draw Lewis electron dot diagrams for the following...Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Acetic acid is the active ingredient of vinegar....Ch. 3 - Under certain conditions, the stable form of...Ch. 3 - White phosphorus (P4) consists of four phosphorus...Ch. 3 - Draw Lewis electron dot diagrams for the following...Ch. 3 - Draw Lewis electron dot diagrams for the following...Ch. 3 - Draw Lewis diagrams for the two resonance forms of...Ch. 3 - Draw Lewis diagrams for the three resonance forms...Ch. 3 - Methyl isocyanate, which was involved in the...Ch. 3 - Prob. 56PCh. 3 - Draw Lewis diagrams for the following compounds....Ch. 3 - Draw Lewis diagrams for the following ions. In the...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - For each of the following molecules or molecular...Ch. 3 - For each of the following molecules or molecular...Ch. 3 - Give an example of a molecule or ion having a...Ch. 3 - Give an example of a molecule or ion having a...Ch. 3 - For each of the answers in Problem 59, state...Ch. 3 - For each of the answers in Problem 60, state...Ch. 3 - Prob. 67PCh. 3 - Mixing SbCl3 and GaCl3 in a 1:1 molar ratio (using...Ch. 3 - (a) Use the VSEPR theory to predict the structure...Ch. 3 - Ozone (O3) has a nonzero dipole moment. In the...Ch. 3 - Assign oxidation numbers to the atoms in each of...Ch. 3 - Prob. 72PCh. 3 - Prob. 73PCh. 3 - Prob. 74PCh. 3 - Prob. 75PCh. 3 - Prob. 76PCh. 3 - Prob. 77PCh. 3 - Prob. 78PCh. 3 - Prob. 79PCh. 3 - Prob. 80PCh. 3 - Prob. 81PCh. 3 - Prob. 82PCh. 3 - Prob. 83PCh. 3 - Prob. 84PCh. 3 - Prob. 85APCh. 3 - Prob. 86APCh. 3 - At large interatomic separations, an alkali halide...Ch. 3 - Prob. 88APCh. 3 - Prob. 89APCh. 3 - Two possible Lewis diagrams for sulfine (H2CSO)...Ch. 3 - There is persuasive evidence for the brief...Ch. 3 - The compound SF3N has been synthesized. (a) Draw...Ch. 3 - Prob. 93APCh. 3 - The molecular ion S3N3 has the cyclic structure ...Ch. 3 - Prob. 95APCh. 3 - Prob. 96APCh. 3 - Prob. 97APCh. 3 - Prob. 98APCh. 3 - A stable triatomic molecule can be formed that...Ch. 3 - The gaseous potassium chloride molecule has a...Ch. 3 - (a) Predict the geometry of the SbCl52 ion, using...Ch. 3 - Prob. 102APCh. 3 - Predict the arrangement of the atoms about the...Ch. 3 - Prob. 104APCh. 3 - Prob. 105APCh. 3 - Prob. 106APCh. 3 - Prob. 107APCh. 3 - Prob. 108APCh. 3 - (a) Determine the oxidation number of lead in each...Ch. 3 - Prob. 110APCh. 3 - Prob. 111CPCh. 3 - Prob. 112CPCh. 3 - A compound is being tested for use as a rocket...Ch. 3 - Prob. 114CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the correct Lewis structure and assign a formal charge to each atom in fulminate ion, CNO.arrow_forwardA stable triatomic molecule can be formed that containsone atom each of nitrogen, sulfur, and fluorine. Threebonding structures are possible, depending on which is thecentral atom: NSF, SNF, and SFN.(a) Write a Lewis diagram for each of these molecules,indicating the formal charge on each atom.(b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom?(c) Does consideration of the electronegativities of N, S,and F from Figure 3.18 help rationalize this observedstructure? Explain.arrow_forwardDraw Lewis structures for HFO4, HFO3, HC0O4, HC0O3, HCO2. (These molecules have the halogen atom as the central atom. All O atoms are attached to the halogen. The hydrogen atom is bonded to one of the O atoms.) Use formal charges to determine which molecule is least likely to occur in nature. (A) HFO4 (B) HC(O2 (C) HC!O3 (D) HFO3 (E) HC\O4 DO000arrow_forward
- Consider the formate ion, HCO2", which is the anion formed when formic acid loses an H* ion. The H and the two O atoms are bonded to the central C atom. (a) Draw the best Lewis structure(s) for this ion. (b) Are resonance structures needed to describe the structure? Explain briefly (c) Would you predict that the C-O bond lengths in the formate ion would be longer or shorter relative to those in CO2? Explain brieflyarrow_forward(a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe—O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in (a) yields the most favorable formal charges for the molecule?arrow_forwardDraw ALL THREE (3) possible Lewis structures for FNO (N is the central atom) and identify the dominant structure using formal charges.arrow_forward
- Draw resonance structures for the bicarbonate ion, HCO3-. (a) Does HCO3- have the same number of resonance structures as the CO32- ion? Are any less likely than others? (b) What are the formal charges on the O and C atoms in HCO3- ? What is the average formal charge on the O atoms? Compare this with the O atoms in CO32- . (c) Protonation of HCO3- gives H2CO3. How do formal charges predict where the H+ ion will be attached?arrow_forwardCyanogen (CN)2 is known as pseodohalogen because it has some properties like halogens. It is composed of two CN’s joined together.(i) Draw the Lewis structure for all the possible combination for (CN)2.(ii) Calculate the formal charge and determine which one of the structures that you have drawn is most stable.(iii) For the stable structure, determine the geometry around the two central atoms.(iv) For the stable structure, draw the dipole arrows for the bonds.(v) Base on the stable structure, determine the polarity of molecule and state your reason.arrow_forwardFinish the following questions. ((a) Draw all of the possible Lewis structures (including reasonance structures) of the following compounds.(b) Label the formal charge for each atom.(c) Determine which resonance structure(s) is(are) the better/best and briefly explain. ClO2F2+arrow_forward
- Three resonance structures are possible for the thiocyanate ion, SCN-. (a) Draw the three resonance structures. (b) Calculate the formal charge on each atom in each resonance structures. (c) Based on formal charges and electronegativity, predict which resonance structure most closely approximates the bonding in this ion? (d) What are the similarities and differences of bonding in SCN compared to the bonding in OCN- .arrow_forwardSome chemists believe that satisfaction of the octet rule should be the top criterion for choosing the dominant Lewis structure of a molecule or ion. Other chemists believe that achieving the best formal charges should be the top criterion. Consider the dihydrogen phosphate ion, HaPO, , in which the H atoms are bonded to O atoms. (a) What is the predicted dominant Lewis structure if satisfying the octet rule is the top eriterion? (b) What is the predicted dominant Lewis structure if achieving the best formal charges is the top criterion?arrow_forwardDraw Lewis diagrams for the following ions. In the formula the symbol of the central atom is given first. (Hint:The valence octet may be expanded for the central atom.)(a) BrO4 - (b) PCl6 - (c) XeF6+arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY